GENFIT Announces Advances Across its ACLF Pipeline at AASLD The Liver Meeting® 2025
Several posters and presentations featuring new data on three ACLF programs, including G1090N (NTZ reformulation) disease model data, new real-world data and biomarker insights in cirrhosis
New Iqirvo® PBC and PSC data to be presented by partner Ipsen, underscoring its potential in rare cholestatic liver diseas
About GENFIT
GENFIT, a BCorp™ certified company since 2025, is a biopharmaceutical company committed to improving the lives of patients with rare, life-threatening liver diseases whose medical needs remain largely unmet. GENFIT is a pioneer in liver disease research and development with a rich history and a solid scientific heritage spanning more than two decades
Today, GENFIT focuses on Acute on-chronic Liver Failure (ACLF) and associated conditions such as acute decompensation (AD) and hepatic encephalopathy (HE). It develops therapeutic assets which have complementary mechanisms of action, selected to address key pathophysiological pathways. GENFIT also targets other serious diseases, such as cholangiocarcinoma (CCA), urea cycle disorders (UCD) and organic acidemia (OA).
Its R&D portfolio, covering several stages of development, ensures a constant news flow.

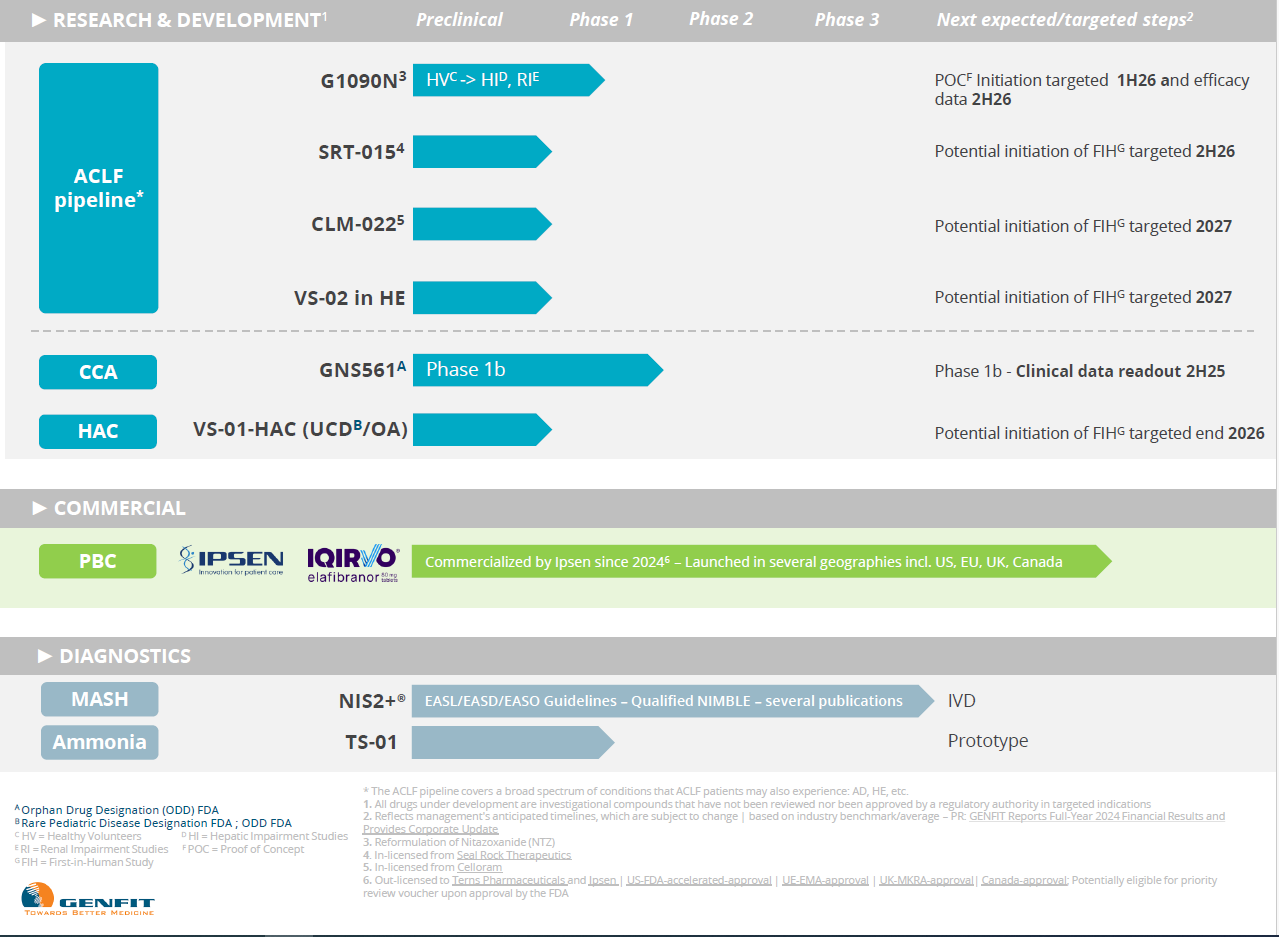
Our Therapeutic Areas
Our R&D pipeline covers targeted therapeutic areas via eight programs across a variety of development stages.
- 4 programs in Acute-on-Chronic Liver Failure (ACLF) and Hepatic Encephalopathy (HE), one of the most common complications of ACLF
- 2 programs in Other Life-Threatening Indications: Cholangiocarcinoma (CCA), and Urea Cycle Disorders (UCD)/Organic Acidemias (OA)
Beyond therapeutics, GENFIT’s pipeline also includes a diagnostic franchise focused on NASH/MASH1 and ammonia.
Our Success in PBC
In June 2023, GENFIT and Ipsen announced positive topline 52-week data from the pivotal Phase 3 trial ELATIVE® evaluating elafibranor in Primary Biliary Cholangitis (PBC). This asset was discovered and successfully developed by GENFIT.
In 2024, Iqirvo® (elafibranor) received Accelerated Approval by the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA) and the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom for the treatment of Primary Biliary Cholangitis (PBC).
Iqirvo® is now marketed in several countries.[1]
[1] Elafibranor is marketed and commercialized, notably in the U.S and Europe, by Ipsen under the trademark Iqirvo®


Our expertise
Our Documentation

Corporate presentation
October 2025
https://ir.genfit.com/static-files/069b79c6-6358-426c-8b59-06226784a333


Latest Press Releases

Our CSR Commitment
We believe that Corporate Social Responsibility (CSR) is a key driver for success. Our positioning in the healthcare sector, at the service of patients, is a central element of our societal commitment, which we complement with proactive social and environmental policies and initiativesdeveloped and carried out by our teams. Our actions are coordinated through a system of corporate governance that meets demanding criteria in terms of ethics, responsibility and fairness.
Our videos
1 At EASL Congress in June 2023 it was announced that nonalcoholic steatohepatitis (NASH) would now be referred to as Metabolic dysfunction-associated steatohepatitis (MASH). Nonalcoholic fatty liver disease (NAFLD) will now be referred to as metabolic dysfunction-associated steatotic liver disease (MASLD). GENFIT is progressively transitioning its documentation over to this new nomenclature and both NASH and MASH terms may appear in our documents during this period
2 GENFIT Communique de presse – 30 juin 2023 : Ipsen et GENFIT annoncent les résultats positifs de l’essai de Phase III ELATIVE® évaluant elafibranor chez des patients atteints de cholangite biliaire primitive, une maladie cholestatique rare du foie